
Above, red crystals of lorándite TlAsS2 pseudomorphing into yellow crystals of fangite Tl3AsS4. The specimen is from one of the world's hotspots for thallium, the Allchar mine, in the Republic of Macedonia. The mine contains a suite of thallium minerals which have a couple of surprising connections to nuclear science...
- The Search for Element 113 (Eka-Thallium) in lorándite
- Use of the lorándite as a solar neutrino detector
The periodic table of the chemical elements was first devised by the particularly scruffy Siberian born Russian chemist Dmitri Mendeleev in 1869. His table listed the (63 then known) chemical elements in rows or columns in order of atomic weight, with a new row or column added when the chemical and physical characteristics of the elements showed similarities.

Above, Mendeleev looks quite smart on this 2009 souvenir sheet featuring a 15 Ruble stamp. His periodic table is behind him, and his interests are represented by a mineral sample from his wonderful collection, chemistry apparatus and a quadrant electrometer. It’s in honour of the 175th anniversary of this great man’s birthday who, even for an audience with his Majesty, Czar Alexander III, refused to have his hair cut.
When Mendeleev published his periodic table of the chemical elements, he noted gaps in the table and predicted that as-then-unknown elements existed with properties appropriate to fill those gaps. He named them eka-boron, eka-aluminium and eka-silicon. These missing elements were eventually discovered in minerals, and found to have physical and chemical properties exactly as the great man (var. unkempt) predicted -
- eka-boron = scandium, found by Lars Fredrik Nilson in the minerals euxenite and gadolinite in 1879
- eka-aluminium = gallium, discovered by Paul Emile Lecoq de Boisbaudran in 1875 from its characteristic spectrum (two violet lines) in a sample of sphalerite
- eka-silicon = germanium, discovered 1886 by Clemens Winkler in the mineral argyrodite Ag8GeS6
More recently the eka- prefix was used by other theorists to temporarily name unknown elements. Element 113, which would occupy an unoccupied space in the periodic table just below thallium, was therefore named eka-thallium. It was way beyond the heaviest (in the sense of size of nucleus) naturally occurring primordial element, uranium (element 92), but it was conjectured that minute amounts of eka-thallium could still exist on earth. This was because eka-thallium nuclei are very near the "Island of Stability," predicted by nuclear physics, and so could have a relatively long half-life -

Soon the search for element 113, eka-thallium, was on. The scientists reckoned that the unknown element would have similar chemical properties to thallium and perhaps could be found in a mineral containing thallium, for instance lorándite.

Lorándite is a thallium arsenic sulphosalt: TlAsS2, forming beautiful cochineal to carmine-red coloured monoclinic crystals. The atomic structure (above) shows thallium atoms as brown, arsenic as violet and sulphur as yellow, arranged as spiral chains of AsS3 tetrahedra interconnected by thallium atoms. These chains are oriented on the [010] axis and have covalent bonding (not shown) between them, linking the irregularly coordinated thallium atoms.
Lorándite was first discovered at the Allchar deposit (the type locality), near Kavadarci, Macedonia in 1894 by Krenner. It was named after Baron Loránd Eötvös, a prominent Hungarian physicist and inventor of the Eötvös torsion balance, a geophysical prospecting instrument -
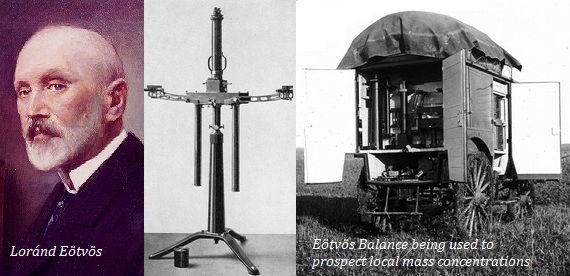
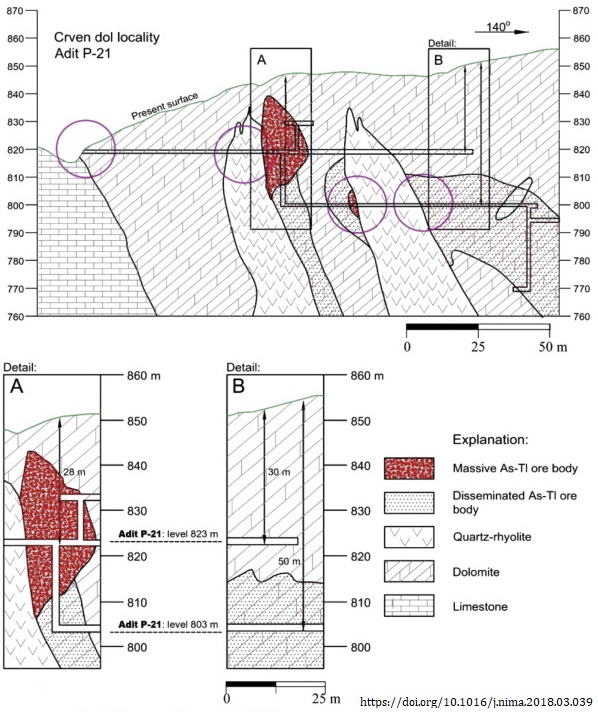
The Allchar (Alšar; Alsar; Alshar) hydrothermal Sb-As-Tl-Au ore deposit has 18 thallium minerals, 11 of which are type locality. The mine still contains around 20 tons of thallium, mostly at the Crven Dol ore body -
Lorándite crystals were taken from the mine in the search for element 113, eka-thallium. It was expected that the element would be highly susceptable to spontaneous fission, and this was hoped to be the signature of the prescence of the element. The researchers' first, and most elegant idea, was to polish and chemically etch a lorándite crystal. This would reveal microscopic fission tracks (damaged lattice along the path of nuclear fission fragments) caused by eka-thallium fission, much like these tracks in apatite, caused by U238 fission -

This proved impossible however, because of the chemical nature of lorándite (see below). Undaunted, the University of Belgrade scientists embarked on a ten year experiment to capture evidence of the fission of eka-thallium.


I wonder whether etching using plasma, laser or cathode-rays (in an inert gas or vacuum) would reveal fission tracks (should they exist) on the surface of a polished lorándite crystal?
Although this research work yielded a negative result, it stands as an excellent example of dogged, solid science - an excellent read. For the eventual discovery of eka-thallium, we must travel from Macedonia to Japan, later in this article. But now we're going solar....
The Sun - A Thermonuclear Furnace
The energy pouring from the sun comes primarily from the proton–proton chain reaction. That is, the fusion of four hydrogen nuclei, which results in a helium nucleus and a couple of neutrinos - on a huge scale. We feel the heat and see the light. But we don't feel the massive flux of sub-atomic particles released by the fusion reactions. Each second, every square centimeter of your body facing the sun, is penetrated by 65 billion neutrinos. Even at night, with the Earth between your body and the sun. Neutrinos go through matter as though it almost wasn't there....
Detecting Solar Neutrinos
Very, very occasionally neutrinos interact with matter. To detect such events requires apparatus costing millions. Huge underground tanks of ultrapure water are used as a target. When an interaction occurs, Cherenkov light is emitted from the resultant shower of electrons and muons. The Cherenkov flashes are detected by arrays of photomultiplier tubes. We'll take a look at such a detector on our visit to Japan later on, but staying in Macedonia for now....
In 1976 it was proposed to use the mineral lorándite as a geochemical neutrino detector. M.K. Pavićević (University of Belgrade) et al proposed the project “Application of Thallium Minerals as Solar Neutrino Detectors”.
This became known as LOREX (the LORandite EXperiment). Thallium has a low threshold energy of 52 keV; in other words it can absorb a neutrino a little easier than gold, for instance. When struck by a neutrino, thallium Tl-205 is transmutated into an isotope of lead, Pb-205
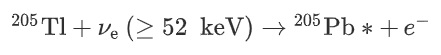
Crystals from the Crven Dol ore body at Allchar were carefully chosen and the lorándite analysed to find the Pb-205 / Tl-205 ratio. Knowing the age of the deposit, 4.31 million years, the mean solar pp-neutrino flux, i.e. the mean luminosity of the sun during the last 4.31 million years can be calculated. The LOREX experimental details are well worth a read, and can be fully accessed here.
The Allchar Mine
Before leaving for The Land of the Rising Sun, here's a photo of the main adit, followed by a few thallium minerals from the Allchar mine.


The commercial output of the mine, i.e. minerals not extracted for nuclear physics or mineral collectors, were processed to make rat-poison. Thallium and arsenic, the main consituents of lorándite, are of course extremely toxic.

Some thallium was used to produce Clerici solution. Spinel, garnet, diamond, and corundum all float on the surface of a solution of thallium formate and thallium malonate. This highly dense liquid was used in mineralogy and gemology for determining crystal density, before its very toxic nature deterred folk from its general use -
Another use for the thallium from Allchar (of interest to mineralogists, unlike its adoption as inheritance powder) is in scintillation detectors. Much more sensitive than geiger counters, they are used to prospect for uranium and thorium minerals, and to perform gamma spectroscopy to determine the isotopes present. Ionizing radiation causes flashes of light within a crystal of sodium iodide, doped with thallium -
There follows a couple of videos concerning the Allchar mine. Unfortunately they're narrated in the Macedonian Language. Both are interesting without audio, but to understand the dialogue, one must realise that Macedonian has only five short vowel phonemes, but a fairly rich consonantal system of 28 phonemes; stress is placed on the first syllable of bi-syllabic words and on the antepenult in words of three or more syllables.
With this in mind, and having the foreign language qualifications of French O-Level (fail), and re-sit French O-Level (fail), I was able to translate the first word of the following dialogue; the lady says "Allchar."
In this next video, mineral collectors are apparently being hassled -
Eastward bound, for nipponese neutrinos...
 Takeo Ito's Japanese Minerals in Pictures, Japanese Japan-law twin quartz, Itai-itai disease victim
Takeo Ito's Japanese Minerals in Pictures, Japanese Japan-law twin quartz, Itai-itai disease victim
Atoms of Eka-Thallium in Japan
Element 113 is a superheavy synthetic element, produced by the Japanese by bombarding Bismuth-209 with Zinc-70. The first few atoms were synthesised in 2004 by a team of scientists at Riken in Wakō, Japan. Eka-thallium was named nihonium, with the symbol Nh. The most stable isotope found so far is Nh-286, with a half-life of 8 seconds.
The eka-thallium gap in Mendeleev's periodic table was filled, albeit with the use of particle accelerators costing hundreds of million Yen. The discovery of the synthetic element nihonium was accomplished without any lorándite crystals being sacrificed on the altar of science, so preserving specimens for collectors!
Also the output from Allchar isn't really required for much in the way of neutrino detection these days, courtesy again the Japanese. Their more superior neutrino detector - The Super-Kamiokande, utilises water as its "scintillator", therefore no thallium was used for its 13 thousand photomultiplier tubes.
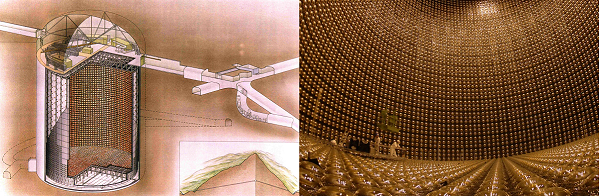
The £270m detector is a tank of 50,000 tons of ultrapure water, 1000m under the peak of Mt.Ikenoyama. The rock overburden reduces cosmic ray muon background radiation. It is located within the old Kamioka mine workings of the Mozumi ore deposit, discovered in 1589 in Gifu, Japan.

"The Kamioka mine is the largest Zn-Pb-Ag mine in Japan and has a production history of about 1,100 years. The ore reserves, are about 100 million tons, including mined ores of 65 million tons that have an average grade of 0.82% Pb, 5.2% Zn and 33g/t Ag (KANO et al., 1992). This may be the largest skarn-type Zn-Pb-Ag deposit in the world (EINAUDI et al., 1981)." Ore Formation Processes of the Mozumi Skarn-type Pb-Zn-Ag Deposit in the Kamioka Mine, Mariko et al.
Toxicology
As an end note, it's interesting to compare the toxicological impact of these two mines used for neutrino research. In terms of size and output, the Macedonian Allchar mine is absolutely dwarfed by the Kamioka mine. In terms of mineral toxicity, Allchar wins hands down. However, apart from a lot of dead rats and a few husbands bumped off, the Macedonian mine appears to be fairly benign.
The Mozumi Pb-Zn-Ag deposit was responsible for the mass cadmium poisoning of the inhabitants of Toyama Prefecture, Japan, starting around 1912.
The locals began to fall ill with distressing symptoms. They called it "itai-itai disease" (translated - "it hurts - it hurts") for the severe pains sufferers felt in the spine and joints. Debilitating softening of the bones and kidney failure eventually led to death. It was cadmium poisoning. The Japanese Ministry of Health and Welfare acknowledged this in 1968....
Cadmium leached into Kamioka mine-water and ore-processing run-off, straight into the Jinzū River. Which irrigated rice paddies for 30 km downstream of the mine.
Having closed the mine, the Japanese government decided to clean the mine water. Deep within the Kamioka mine, a purification plant was set up to process mine-water into ultrapure water. UPW is water that has been purified to uncommonly stringent specifications. Much, much purer than deionized or distilled water, ultrapure water has the highest levels of purity for all contaminant types, including: organic and inorganic compounds; dissolved and particulate matter; volatile and non-volatile, reactive and inert; hydrophilic and hydrophobic; and dissolved gases, particularly radon.
Of course none of this water hit the rice paddies, it was needed for science! The environmental catastrophe at Toyama Prefecture had been apparently dealt with, at a cost of nearly £290 million for compensation and to reduce Cd levels in the topsoil. The mine owners, Mitsui Mining and Smelting, had managed to reduce the amount of cadmium they were discharging into the Jinzū river. From an incredible 35kg per day in 1972, to "only" 5kg per day in 1992.
It is odd to think that the same mine produced both some of the world's most polluted water, and the world's most pure...
References -
A Search for the Element 113 in Nature, Antanasjević et al, 1994
Lorandite from Allchar as geochemical detector for pp-solar neutrinos. Pavićević et al, 2018
Allchar Mineral Assemblage, Boev et al., Geologica Macedonica, 2001
Ore Formation Processes of the Mozumi Skarn-type Pb-Zn-Ag Deposit in the Kamioka Mine, Mariko et al., 1996
And Now For Something Completely Different