
When water freezes, it forms the mineral ice. The most beautiful specimens soon melt, so e-Rocks has never sold any. Don’t be dismayed by the prospect of an article on ice though, just wade through a next bit about snowflakes, then the post livens up with giant iron crystals, cameras transported at 51000 MPH, catastrophic feedback, and natural mercury crystals.
Perhaps the wrong time to mention snow, as the British heatwave continues, but Winter has its attributes, quite apart from Santa emptying his sack all over your living-room carpet. Snowflakes are perhaps the most beautiful natural crystals a mineralogist can examine. They’re of an ephemeral nature, and collecting these fragile mineral crystals for a collection is doomed to failure. At STP (Standard Temperature and Pressure, 273 K (0° Celsius or 32° Fahrenheit), 1 atm pressure), the crystals don’t melt, but slowly sublime to water vapour, the reverse of their formation in the atmosphere. It is possible to “preserve” snowflakes, but these are artificial organic (cyanoacrylate) epimorphs. The process is excellently described at snowflakes.com, a website well worth a visit, containing a plethora of information on the subject. It’s by Prof. Kenneth Libbrecht at Caltech, here’s a video of him and some of his growing crystals -
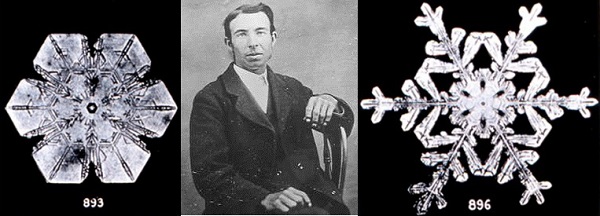
Snowflakes were first photographed by Wilson Alwyn "Snowflake" Bentley (1865 - 1931), above. He took pictures of some 5000 flakes and, by the look of his fingernails, suffered for his art!

The first scientific research on the growth of ice crystals was undertaken by Japanese physicist Ukichiro Nakaya (1900-1962). Shown above in his low-temp lab wearing his fur-lined lab-attire, growing snowflakes. He became obsessed by ice crystals, and famously wrote in 1939, “Snowflakes are letters sent from heaven." in his seminal work Snow Crystals: Natural and Artificial. His work led him to create the Nakaya Diagram below, which shows the variety of morphology of ice crystals, at various temperatures & humidity-


Above, beautiful SEM (scanning electron-microscope) images of ice crystals. The saying that “no two snowflakes are the same” is actually incorrect. Lab grown crystals can be identical in form, given constant conditions. Natural snowflakes have a complex genesis within clouds, mixing and individually travelling through areas of a cloud having different temperatures and humidities. Here’s a couple of flakes Nakaya photographed; the artificial one is growing on a rabbit hair, his preferred matrix -
Snowflakes have been described forming 121 different types, all following a basic hexagonal symmetry. Ice forming on water yields even more morphologies, but all structures are based on how the water molecules bind together -
Snow can compact and recrystallise into glacial ice. Here’s an excellent 48 second video of the movement of this mineral -
Large ice crystals grow from fumarolic gas issuing from the Mt. Erebus volcano in Antarctica. The gases have melted out spectacular ice-caves, in which beautiful crystals sublime out. The following photo (
by Nial Peters) is from an excellent article, “
Erebus science – ice caves” at volcanofiles.com. It shows 15cm hexagonal specimens -

OK, that’s enough concerning solid water,.. for now…. Taking a tangential track, I’d like to look at another, even more abundant mineral, with crystals that offer challenging storage problems.
Really BIG crystals are about 3160 miles from you at the moment. Huge iron crystals, aligned north to south, with dimensions measured in miles. The inner core of the Earth is known only through seismic and magnetic measurements at the surface and, using these data, modelling in supercomputers. The inner core is a solid mass of mainly iron, with a radius of 760 miles. The interpreted data suggests that it is white-hot, hexagonal close-packed crystalline iron, with all crystals aligned. It’s a fascinating subject.
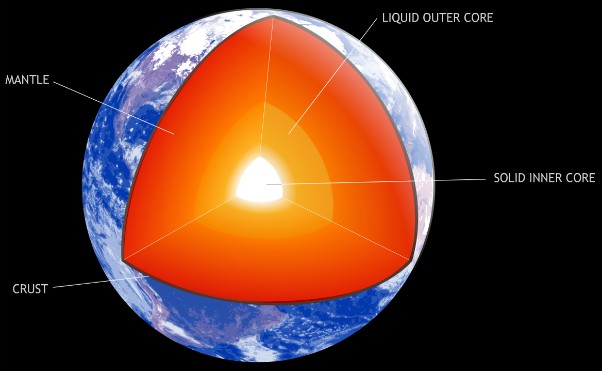
Aside from the sheer bulk of these crystals, and extremely difficult access for extraction, the storage of such specimens would offer severe problems. Keeping a snowflake is easy in comparison - a chamber precisely holding a constant sub-zero temperature, and absolutely stable humidity, to prevent any danger of sublimation. The chamber for an iron crystal would have to maintain a temperature of 5,430 °C (about that of the sun’s surface), and a pressure of 3,500,000 atmospheres. Failure of the chamber would result in the crystal violently exploding into white-hot iron plasma, with the force of a meteorite impact. This could be dangerous to bystanders.
The Earth’s inner core will remain inaccessible to mineral collectors. Not so, crystals at the other extreme of temperature and pressure; Mile-wide ice crystals? On a planet smaller than the Earth’s inner core?.... In 2007 a very expensive camera was propelled by a Lockheed Martin Atlas V 551 rocket, and the gravity assist of the planet Jupiter, to an incredible speed of 51000 mph. It was on its way to Pluto, which has a radius of 738 miles.
The camera, the Long-Range Reconnaissance Imager (LORRI) is equipped with a 1024×1024 pixel by 12-bits-per-pixel monochromatic CCD imager. It is powered by 21.5 lbs of plutonium 238 in an RTG (Radioisotope Thermoelectric Generator). The camera is part of the scientific payload of the New Horizons spacecraft, together with an ounce of human remains - the ashes of Clyde Tombaugh.


The New Horizons spacecraft, the black finned cylinder is the power source, the RTG. The discoverer of Pluto next to his telescope, technicians fitting the LORRI camera, a slug of glowing plutonium for the RTG, and lift-off on January 19, 2006
The images returned to Earth in 2015 were of a totally unexpected nature. Most preconceived ideas about the dwarf planet Pluto, were it to be a static, disappointing cratered ball of ice. What was revealed was a complex, dynamic morphology of nitrogen ice glaciers, convective cells and possible cryovolcanoes. Mountain ranges of water ice. Kilometer high blades of methane ice. “Sand” dunes of methane and nitrogen ice grains. When I first saw the images released by NASA it did strike me that some of the mountain ranges reminded me of crystal aggregates. What do you think?
Swiftly returning to Earth….

Above, one of the most dangerous minerals on Earth. Few collectors have a specimen, even though it is abundant. It’s methane clathrate. Here’s some recently collected samples of ice that burns -


Methane clathrate, also known as methane hydrate or fire-ice, is a crystalline water-based solid physically resembling ice, in which single methane molecules are trapped inside "cages" of hydrogen bonded, frozen water molecules. Without the support of the trapped methane molecules, the lattice structure of methane hydrate would collapse into conventional ice crystal structure or liquid water. It’s not officially a chemical compound, as the sequestered non-polar methane molecules are never bonded to the lattice.

There’s a LOT of methane clathrate about - Around 6.4 trillion tonnes of methane is trapped in deposits of methane clathrate on the deep ocean floor. It also occurs in permafrost on land.
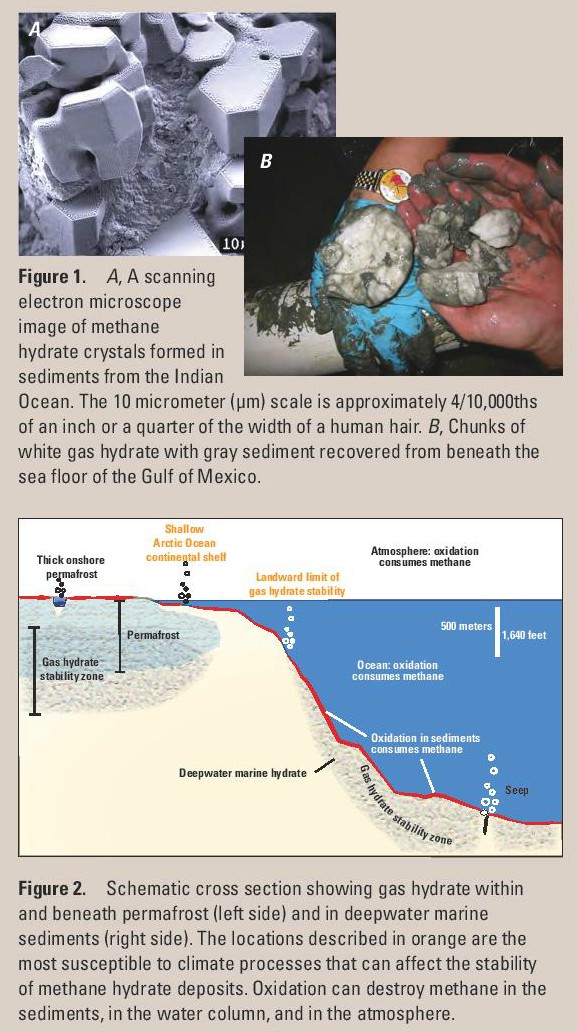
Above from Gas Hydrate in Nature USGS public domain document
If the pressure is reduced, or the temperature raised, methane hydrate melts and releases methane gas. Collectors have to maintain constant refrigeration of their specimens. More of a worry is the fragile nature of the huge in-situ deposits of this mineral. The sudden release of large amounts of gaseous methane from long frozen methane clathrates has been implicated in the major extinction event at the Permian-Triassic boundary 250 mya. Many of the deposits shown on the following map are on the “ring of fire,” and a violent readjustment of sea-bed level in these seismically active areas would be catastrophic. As would continued global warming. Either events could cause positive feedback, the more methane released, the hotter it becomes (methane is a very potent greenhouse gas), more methane hydrates undergo phase transition, releasing more greenhouse gas. This is known as the Clathrate gun hypothesis

A slightly more benign mineral is quicksilver, or natural mercury. It occurs in, and can seep out of, cinnabar deposits. The quicksilver at Idria Mine (Idrija Mine) in Slovenia was discovered by such a seep -
"The great mine at Idria is situated in the body of a mountain; and it is said that it was discovered nearly three centuries back by the trickling out of the native mercury from a fissure through which a small spring discharged itself. The mines at Idria are in the possession of the Austrian Government, and produce about one hundred and fifty tons of the metal annually."
A Manual of Metallurgy, George Hogarth Makins, 1865
Mercury freezes at -38.8 °C. It can get a bit nippy in various parts of the world, this from The New and complete American encyclopedia, 1807 -
The Freezing of Quicksilver by Natural Cold. The most remarkable congelation of mercury, by natural cold, that has ever been observed, was that related by Dr. Peter Simon Pallas [of Pallasite meteorite fame], who had been sent by the empress of Russia, with some other gentlemen, on an expedition similar to that of D. Gmelin. Being at Krasnoyarsk, in 1772, in N. lat. 56° 30', and E. long. 95°, he had an opportunity of observing this phenomenon, “On the 6th and 7th of December, I placed upon the gallery, on the N. side of my house, some quicksilver in an open bowl. Within an hour I found the edges and surface of it frozen solid; and some minutes after the whole was condensed by the natural cold into a soft mass very much like tin. While the inner part was still fluid, the frozen surface exhibited a great variety of branched wrinkles; but in general it remained pretty smooth in freezing. An instance of the natural congelation of quicksilver also occurred in Jepthland, a province of Sweden, on the 1st January, 1782; and on the 26th, Mr. Hutchins observed the same effect of the cold at Hudson's bay; when he found at the point of its freezing a mercurial thermometer stood at 40", and a spirit thermometer at 30".”
So… natural mercury crystals could exist, in-situ. It would be their storage, at less than -38.83 °C, which would cause the mineral collector no end of stress, after the jubilation of discovery! A subject touched upon in the chapter, “Slowsilver Discovery North-West of Kyzyl” in my novel, “The Ernest Glitch Chronicles.”
Lastly, and breaking news, the Hayabusa 2 spacecraft has just sent pictures of the 0.6 mile wide asteroid Ryugu, from about 12 miles away. Ryugu is an object of the rare spectral type Cg, with qualities of both a C-type asteroid and a G-type asteroid. Its spectra is very similar to those of carbonaceous chondrite meteorites.

Yuichi Tsuda, Hayabusa 2’s project manager wrote, “The shape of Ryugu is now revealed, from a distance, Ryugu initially appeared round, then gradually turned into a square before becoming a beautiful shape similar to fluorite... Now, craters are visible, rocks are visible and the geographical features are seen to vary from place to place… This form of Ryugu is scientifically surprising…”
Well Yuichi, I would have said the apparent octahedral form is more reminiscent of a diamond. But this is because I’m more familiar with fluorite crystallising as cubes, being a North Pennines collector, and unfortunately not because of familiarity with the desirable carbon allotrope.
The Japanese space agency (JAXA) haven't pulled any punches with the design of Hayabusa 2. It’s a robotic mineral collector with extreme prejudice. Having surveyed the asteroid from fly-pasts, and the deployment of 4 rovers, the spacecraft will approach and touch the surface with its horn.

Hayabusa’s horn (above JAXA / Akihiro Ikeshita) will fire a 5 gram tantalum bullet into the asteroid’s surface at 300 meters per second. The impact ejecta flying from under the surface is caught and collected by a catcher, to be returned to Earth. Not exactly Estwing collecting tools. It gets more brutal. The Japanese don’t mess about - a shaped charge of the high-explosive HMX (4.5kg!) will drive 2.5kg of copper, in the form of a hypersonic-velocity explosively formed penetrator, into Ryugu. This will form a new crater on the asteroid, and Hayabusa will rummage in it for more samples.
The specimens will be in the hands of scientists in December 2020. Well, not in their hands… I would hope that the environmental chamber for the samples will recreate the conditions in space, just to prevent degradation and outgassing of organics (such as happened to much material from the Tagish Lake carbonaceous chondrite meteorite). Ryugu minerals present more of a storage problem than snowflake specimens!
References -
The Freezing of Quicksilver by Natural Cold, New and complete American encyclopedia, 1807
A Manual of Metallurgy, George Hogarth Makins, 1865
Gas Hydrate in Nature USGS, 2018
Erebus science – ice caves Aaron Curtis & Volcanofiles, 2011
And Now For Something Completely Different